Introduction: Hemophilia A is a rare bleeding disorder caused by pathogenic variants in the F8 gene, resulting in insufficient factor VIII (FVIII) activity. Adeno-associated virus (AAV)-mediated gene transfer enables the delivery of a modified functional F8 gene to hepatocytes that subsequently synthesize FVIII at levels that may prevent bleeding events in the absence of exogenous FVIII. Updated results and follow-up from the Alta study, an ongoing gene therapy study in patients with severe hemophilia A, are presented.
Methods: The Alta study is a phase 1/2 dose-ranging, single-dose study of giroctocogene fitelparvovec (also known as SB-525 and PF-07055480), a recombinant AAV serotype 6 (rAAV6) vector encoding a modified F8 gene. Adults aged ≥18 years with severe hemophilia A were eligible for inclusion. Giroctocogene fitelparvovec was infused into patients in 4 cohorts of 2 patients each across 4 ascending doses (9e11, 2e12, 1e13, and 3e13 vg/kg). The 3e13 vg/kg dose cohort was expanded with 3 additional patients. Key end points included safety, circulating FVIII activity, use of FVIII replacement therapy, and frequency of bleeding events. Presented data are from the ongoing Alta study (NCT#03061201; data cutoff date, 26 May 2020; database not locked; data reflect those at time of data cutoff, have not undergone standard quality checks, and may be subject to change).
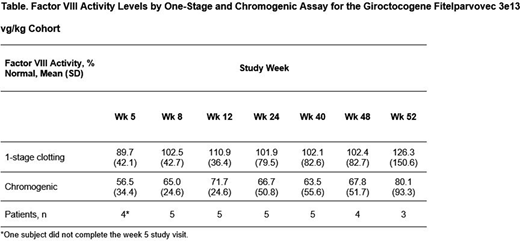
Results: Eleven male patients participated in the study (mean [SD] age, 30.3 [7.8] years; white, 81.8%). As of the cutoff date, patients have been followed for 35 to 144 weeks; one patient in the 1e13 vg/kg cohort discontinued from the study. Overall, the most commonly reported adverse events (AEs; n) included increased alanine aminotransferase (ALT; 8 [72.7%]), increased aspartate aminotransferase (AST; 5 [45.5%]), upper respiratory tract infection (4 [36.4%]), and pyrexia (4 [36.4%]). Treatment-related serious AEs were reported in 1 patient (in the 3e13 vg/kg cohort) who experienced hypotension and fever ≈6 hours after giroctocogene fitelparvovec infusion; the events fully resolved with treatment and did not delay post-infusion discharge. In the 3 lower-dose cohorts, no ALT elevation requiring more than 7 days of corticosteroid treatment was observed. Of the 5 patients in the 3e13 vg/kg cohort, 4 had elevations in ALT that were managed with a tapering course of corticosteroids (ranging from 10-134 days) without loss of clinically relevant FVIII activity through 40 weeks, as evidenced by a lack of bleeding events before and after treatment with corticosteroids. Increases in FVIII activity from baseline were generally dose-dependent. Patients in the 3e13 vg/kg cohort achieved a mean normal-range of FVIII activity within 5 weeks post-infusion, with mean FVIII activity maintained through week 40, which is the last time point with data for all 5 patients in this cohort (Table). Following the initial prophylactic period of up to ≈3 weeks after giroctocogene fitelparvovec administration, no bleeding events occurred in any patient treated in the 3e13 vg/kg cohort. Use of FVIII replacement therapy ≥3 weeks after giroctocogene fitelparvovec administration was reported in 5/6 patients in the lower-dose cohorts (range: 9-115 infusions); none of the patients in the 3e13 vg/kg cohort required FVIII replacement beyond initial use of prophylactic factor for up to ≈3 weeks (prophylactic coverage stopped 3 weeks and 2 days after giroctocogene fitelparvovec administration in 1 patient in the 3e13 vg/kg cohort).
Conclusions: To date, a single infusion of giroctocogene fitelparvovec gene therapy in patients with severe hemophilia A resulted in dose-dependent and sustained increases in FVIII levels without administration of exogenous FVIII, bleeding episodes or sustained adverse events in the highest-dose cohort (3e13 vg/kg). Additionally, patients treated in the highest-dose cohort achieved a mean FVIII activity in the normal range within 5 weeks, which was maintained through week 40. Data on all patients with more than 1 year of follow-up will also be presented. The study is ongoing, and these interim results support further development of giroctocogene fitelparvovec for the treatment of patients with severe hemophilia A.
Leavitt:BioMarin: Membership on an entity's Board of Directors or advisory committees. Konkle:Sanofi: Consultancy, Research Funding; Takeda: Research Funding; Uniquire: Research Funding; CSL Behring: Consultancy; BioMarin: Consultancy; Baxalta: Research Funding; Spark: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Sigilon: Consultancy, Research Funding; Roche: Consultancy. Stine:Biomarin: Consultancy; Applied Stem Cell Therapeutics: Consultancy. Visweshwar:Biogen Idec: Membership on an entity's Board of Directors or advisory committees. Giermasz:uniQure: Consultancy, Research Funding; Sangamo Therapeutics: Research Funding; Bioverativ/Sanofi: Consultancy, Research Funding, Speakers Bureau; BioMarin: Consultancy, Research Funding, Speakers Bureau; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau. Arkin:Pfizer: Current Employment, Current equity holder in publicly-traded company, Other: own stock/options in the company. Fang:Pfizer Inc.: Current Employment, Other: own stock/options in the company. Plonski:Pfizer Inc.: Current Employment, Other: own stock/options in the company. Smith:Pfizer Inc.: Current Employment, Other: own stock/options in the company. Tseng:Pfizer Inc.: Current Employment, Other: own stock/options in the company. Di Russo:Pfizer Inc.: Current Employment, Other: own stock/options in the company. Cockroft:Sangamo Therapeutics: Current Employment, Other: Shareholder of Sangamo Therapeutics. Rupon:Pfizer Inc.: Current Employment, Other: own stock/options in the company. Rouy:Sangamo Therapeutics: Current Employment, Other: Shareholder of Sangamo Therapeutics.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal